Home > Products > Herbal Extract > 6-80% Oleuropein (HPLC) 10-30% Hydroxytyrosol Olive Leaf Extract Plant Extract
6-80% Oleuropein (HPLC) 10-30% Hydroxytyrosol Olive Leaf Extract Plant Extract
PRODUCT DETAILS Product Name: Olive leaf extract Latin Name: Olea europaea L Specification: 6-80% Oleuropein, 10-30% Hydroxytyrosol Test Method: HPLC Part of used: Leaf Appearance:Yellow Brown powder TECHNICAL DATE SHEET ......
Send Inquiry
Product Description
PRODUCT DETAILS
Product Name: Olive leaf extract
Latin Name: Olea europaea L
Specification: 6-80% Oleuropein, 10-30% Hydroxytyrosol
Test Method: HPLC
Part of used: Leaf
Appearance:Yellow Brown powder
TECHNICAL DATE SHEET
| TECHNICAL DATE SHEET | ||
| PRODUCT SPECIFICATIONS | ||
| Product Name: | Olive leaf extract | |
| Botanic Name: | Olea europaea L | |
| Part of plant | Leaf | |
| Solvent extraction | Water & ethanol | |
| Country of Origin: | China | |
| Excipent | 10% maltodextrin | |
| ANALYSIS ITEMS | SPECIFICATION | TEST METHOD |
| Appearance | Fine powder | Organoleptic |
| Color | Yellow Brown powder | Visual |
| Odour & Taste | Characteristic | Organoleptic |
| Identification | Identical to R.S. sample | HPTLC |
| Oleruopein | 6.0%~80.0% | HPLC |
| Sieve Analysis | 100% through 80 mesh | USP39 <786> |
| Loss on drying | ≤ 5.0% | Eur.Ph.9.0 [2.5.12] |
| Total Ash | ≤ 5.0% | Eur.Ph.9.0 [2.4.16] |
| Lead (Pb) | ≤ 3.0 mg/kg | Eur.Ph.9.0<2.2.58>ICP-MS |
| Arsenic (As) | ≤ 1.0 mg/kg | Eur.Ph.9.0<2.2.58>ICP-MS |
| Cadmium(Cd) | ≤ 1.0 mg/kg | Eur.Ph.9.0<2.2.58>ICP-MS |
| Mercury(Hg) | ≤ 0.1 mg/kg -Reg.EC629/2008 | Eur.Ph.9.0<2.2.58>ICP-MS |
| Heavy metal | ≤ 10.0 mg/kg | Eur.Ph.9.0<2.4.8> |
| Solvents Residue | Conform Eur.ph. 9.0 <5,4 > and EC European Directive 2009/32 | Eur.Ph.9.0<2.4.24> |
| Pesticides Residues | Conform Regulations(EC) No.396/2005 including annexes and successive updates Reg.2008/839/CE | Gas Chromatography |
| Aerobic bacteria(TAMC) | ≤1000 cfu/g | USP39 <61> |
| Yeast/Moulds(TAMC) | ≤100 cfu/g | USP39 <61> |
| Escherichia coli: | Absent in 1g | USP39 <62> |
| Salmonella spp: | Absent in 25g | USP39 <62> |
| Staphylococcus aureus: | Absent in 1g | |
| Listeria Monocytogenens | Absent in 25g | |
| Aflatoxins B1 | ≤ 5 ppb -Reg.EC 1881/2006 | USP39 <62> |
| Aflatoxins ∑ B1, B2, G1, G2 | ≤ 10 ppb -Reg.EC 1881/2006 | USP39 <62> |
| Packing | Pack in paper-drums and two plastic-bags inside N.W. 25 kgs I.D.35xH51cm. | |
| Storage | Store in a well-closed container away from moisture, light, oxygen. | |
| Shelf Life | 24 months under the conditions above and in its original packaging | |
PRODUCT FUNCTION
1.Antioxidant effect
2. Bacteriostatic effect
3. Hypoglycemic effect
4. The role of the cardiovascular system
5. Anti-tumor effect
6. Anti-inflammatory effect
7. Antiviral effect
COMPANY PROFILE

FACTORY SHOWS

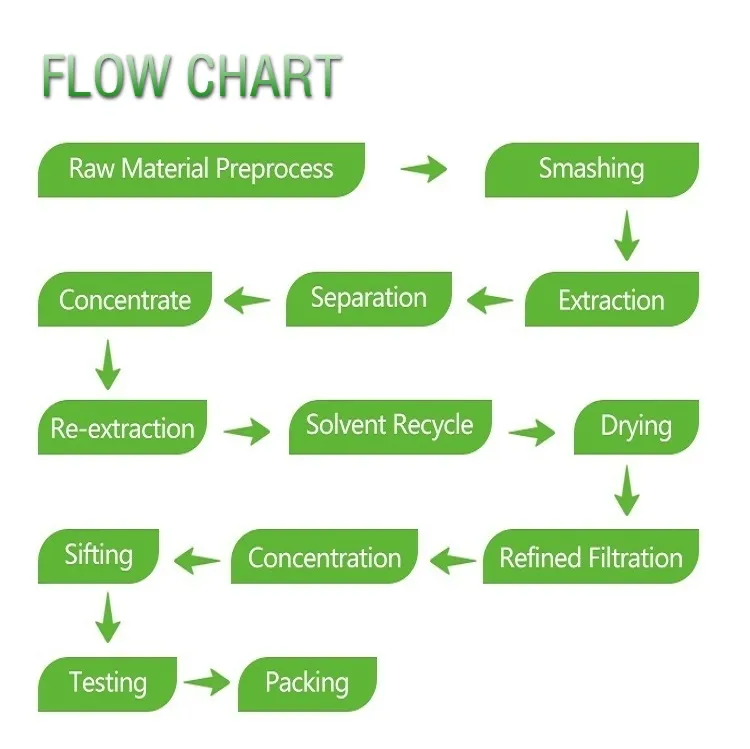
FACTORY PRODUCTION
PACKAGE OF GOODS

SHIPMENT & PAYMENT

Related Category
Send Inquiry
Please Feel free to give your inquiry in the form below. We will reply you in 24 hours.